

Lavoisier's book contained a list of "simple substances" that Lavoisier believed could not be broken down further, which included oxygen, nitrogen, hydrogen, phosphorus, mercury, zinc and sulfur, which formed the basis for the modern list of elements. Lavoisier defined an element as a substance whose smallest units cannot be broken down into a simpler substance. In 1789, French chemist Antoine Lavoisier wrote Traité Élémentaire de Chimie ( Elementary Treatise of Chemistry), which is considered to be the first modern textbook about chemistry. In 1661, Boyle defined an element as "those primitive and simple Bodies of which the mixt ones are said to be composed, and into which they are ultimately resolved." The discovery of phosphorus helped to raise the question of what it meant for a substance to be an element. He kept his discovery secret until 1680, when Anglo-Irish chemist Robert Boyle rediscovered phosphorus and published his findings. In 1669, or later, his experiments with distilled human urine resulted in the production of a glowing white substance, which he called "cold fire" ( kaltes Feuer). Brand tried to discover the philosopher's stone-a mythical object that was supposed to turn inexpensive base metals into gold. The first person in recorded history to discover a new element was Hennig Brand, a bankrupt German merchant.
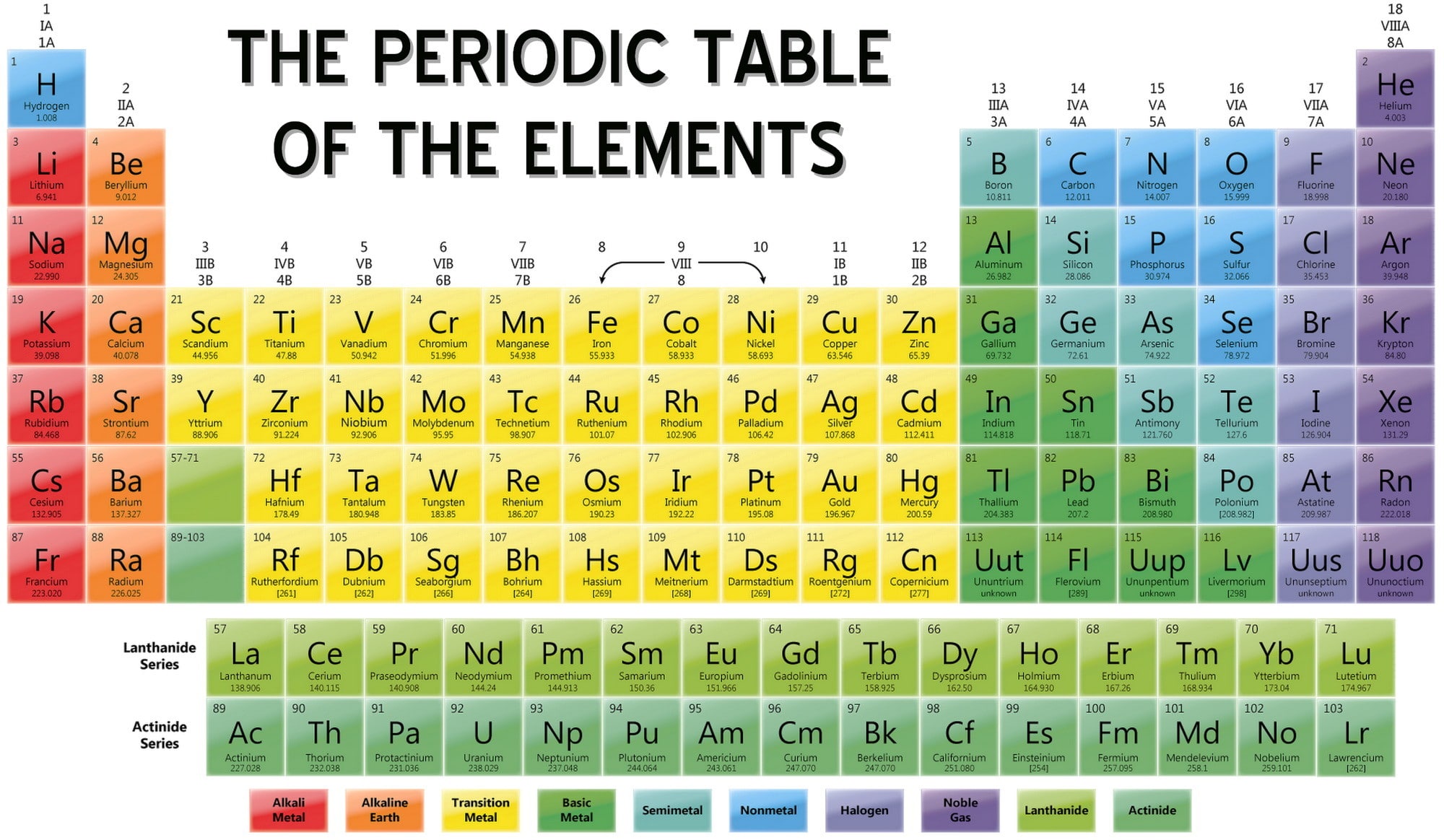
The history of the periodic table is also a history of the discovery of the chemical elements. Hennig Brand, as shown in The Alchemist Discovering Phosphorus


 0 kommentar(er)
0 kommentar(er)
